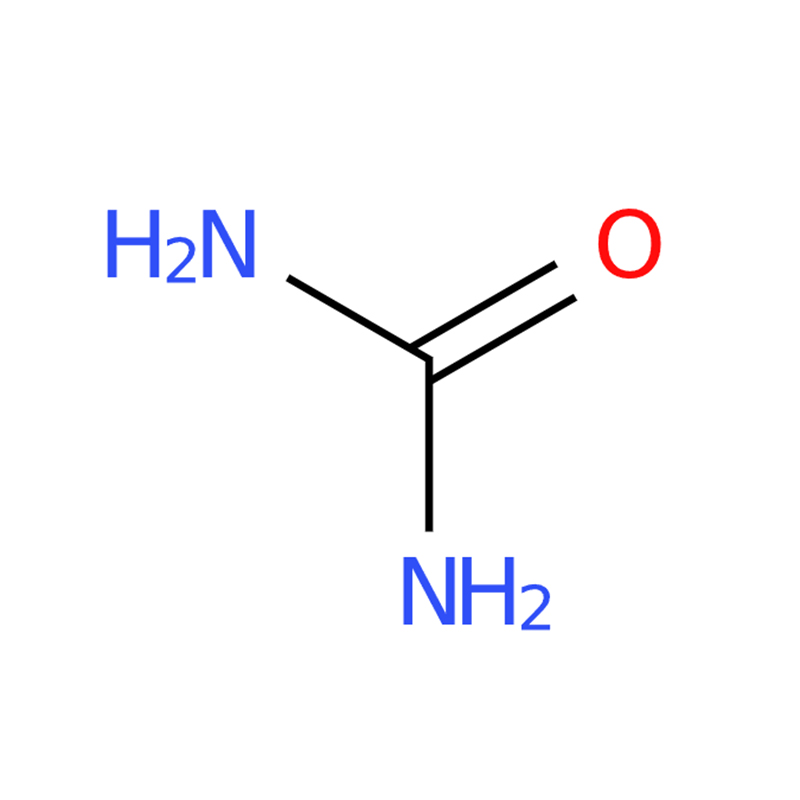
Urea CAS#57-13-6
Molecular Structure: Urea features a planar crystal structure with pyramidal geometry around nitrogen in its gas-phase minimum-energy structure, contributing to its unique properties.
Hydrogen Bonding: In solid urea, the oxygen center forms two N-H-O hydrogen bonds, creating a dense, stable hydrogen-bond network that enhances its molecular stability.
High Aqueous Solubility: Due to its ability to form extensive hydrogen bonds with water, urea exhibits high solubility, making it effective in various industrial and chemical applications.
Chemical Bonding: Urea's carbon is sp2 hybridized, and its C-N bonds have significant double bond character, making it reactive and versatile for use in chemical synthesis.
The urea molecule exhibits a planar structure in its crystal form, while the nitrogen atoms adopt a pyramidal geometry in the gas-phase minimum-energy configuration. In the solid state, the oxygen atom forms two N-H-O hydrogen bonds, creating a dense and energetically favorable hydrogen-bond network. This network likely sacrifices optimal molecular packing, resulting in an open structure with ribbons that form square cross-sectional tunnels. The carbon in urea is sp2 hybridized, giving the C-N bonds notable double bond character, and the carbonyl oxygen is more basic than that in compounds like formaldehyde. Urea’s high solubility in water is attributed to its ability to form extensive hydrogen bonds with water molecules.
Urea Chemical Properties
Melting point | 132-135 °C(lit.) |
Boiling point | 332.48°C (estimate) |
density | 1.335 g/mL at 25 °C(lit.) |
vapor pressure | <0.1 hPa (20 °C) |
refractive index | n20/D 1.40 |
storage temp | 2-8°C |
solubility | H2O: 8 M at 20 °C |
form | |
pka | 0.10(at 25℃) |
color | white |
Specific Gravity | 1.335 |
Odor | almost odorless |
PH | 8.0-10.0 (20℃, 8M in H2O) |
Water Solubility | 1080 g/L (20 ºC) |
λmax | λ: 260 nm Amax: 0.03 |
Merck | 14,9867 |
BRN | 635724 |
Dielectric constant | 3.5(Ambient) |
Stability | Substances to be avoided include strong oxidizing agents. Protect from moisture. |
InChIKey | XSQUKJJJFZCRTK-UHFFFAOYSA-N |
LogP | -1.660 (est) |
CAS DataBase Reference | 57-13-6(CAS DataBase Reference) |
NIST Chemistry Reference | Urea(57-13-6) |
EPA Substance Registry System | Urea (57-13-6) |
Safety Information
Hazard Codes | Xn,Xi |
Risk Statements | 36/37/38-40-38 |
Safety Statements | 26-36-24/25-37 |
RIDADR | Not regulated |
WGK Germany | 1 |
RTECS | YR6250000 |
TSCA | Yes |
HS Code | 31021010 |
Hazardous Substances Data | 57-13-6(Hazardous Substances Data) |
Toxicity | LD50 orally in Rabbit: 8471 mg/kg LD50 dermal Rat 8200 mg/kg |
Urea acts as a physiological regulator of nitrogen excretion in mammals, synthesized in the liver as a byproduct of protein breakdown and excreted in urine. It is also naturally present in the skin. Urea functions as an emollient and diuretic.
It is used to denature proteins and as a mild solubilizing agent for insoluble or denatured proteins. It is particularly useful for renaturing proteins from samples that have been previously denatured with 6 M guanidine chloride, such as inclusion bodies. Urea can also be combined with guanidine hydrochloride and dithiothreitol (DTT) for the refolding of denatured proteins back into their native or active forms.